A CRISPR View of Life
By Shweta Sahu
 |
| Image courtesy of Wikimedia Commons |
We now live in a society where many are trying to get a leg up where they can, whether it be through pharmacological neuroenhancement (like Ritalin and Adderall) or other neurotechnologies (like transcranial direct current simulation). Technology also allows us to exert an even earlier influence on neurodevelopmental disorders through prenatal genetic testing for fetuses. Such technologies include amniocentesis and chorionic villus sampling, that screen for Down’s, Edwards’ and Patau’s syndromes, and give parents the chance to decide whether they would like to terminate or continue with their pregnancy. One article even claims 53% of all pregnancies were aborted following prenatal diagnoses of Down’s Syndrome, though there is still much dispute over the exact numbers.
More recently, research has turned to looking into how to intervene at even earlier stages with gene editing of embryos. CRISPR (clustered regularly interspaced short palindromic repeats) is a naturally occurring bacterial defense mechanism, that when combined with certain enzymes, like “Cas” (CRISPR associated proteins), enable scientists to manipulate the gene sequence of an organism. CRISPR technology brings to life the idea that we can edit genes by either inserting or cutting out specific DNA sequences. Among the vast, exciting biomedical applications of this CRISPR/ Cas system are some promising leads, such as developing CRISPR based disease models. Diseases like schizophrenia and autism involve many genes and using CRISPR, one lab has been able to recreate the genetic mutations and investigate the “faulty” neurons that play a role in these conditions in animal models more efficiently. Whereas this previously took a couple years (requiring the time consuming method of trial-and-error to find the specific sites of mutation) projects like this take only a couple months with the help of CRISPR due to its ability to target sequences in an efficient, site-specific manner.
Moreover, using genome engineering technologies like CRISPR has the potential to increase our ever-growing knowledge of disease processes and subsequent treatments. More specifically, this ability allows us to treat diseases involving muscle differentiation, cancer, inflammation, fetal hemoglobin, and Epstein Barr in animal models and some hope to potentially be used to eliminate gene mutations from the human population. One example of this includes a special birth case. This year, a 3-parent baby was born via a technique called “spindle nuclear transfer”, where mitochondrial genes from a donor woman were added to the mother’s own egg, and consequently fertilized by the father’s sperm. This reproductive technology was prompted by the desire to avoid Leigh syndrome, a fatal neurologic condition affecting one in 40,000 newborns. The mother had previously given birth to 2 babies with this condition, and thus looked to the 3-parent embryo technique as the answer. Because the Food and Drug administration (FDA) banned such a technique in the US, the procedure was performed in Mexico, where no such FDA exists.
Further yet, this CRISPR/ Cas technology may be the first stepping stone to applying these as therapies for neurological diseases. Many genetic disorders of the nervous system are caused by trinucleotide repeats, and recently, the CRISPR/ Cas system has been used in editing the mutations that cause conditions such as Huntington’s Disease (HD) and Fragile X Syndrome (FXS) in cell models. The CRISPR/ Cas system, in conjunction with single guide RNA (sgRNA) targets a specific sequence of DNA and induces a site-specific double stranded DNA break that can subsequently be either altered—resulting in a silenced gene—or deleted—resulting in a null allele. These small cuts in the DNA lead to larger changes in the gene, which affects function of subsequent proteins. When this procedure is applied to mutated genes throughout the genome of an organism, it can restore proper gene/ protein function. In this way, gene editing has the potential to provide the basis for successful therapy for neurodevelopmental disorders.
However, today's debate concerns not so much the research itself, but rather its ethical implications and the clinical applications that result in permanent changes to the human gene pool. For example, one article cites the example of a seven-time Olympic medalist in cross-country skiing whose genetics, the author claims, enabled the medalist to excel in his chosen sport. Might it be possible some athletic-oriented parents wanted the same for their child and genetically edited their child’s genome so as to introduce the overactive erythropoietin gene that confers high oxygen-carrying?
Genetically modifying genes opens doors to another major ethical concern: designing babies that carry permanent allele alterations implies heritable changes to the human germline DNA. Traits that society deems “unfit” would be eliminated from the population by selecting for the “better” alleles of the “fit” population; in turn, these “fit” alleles would be fixed into the population with gene editing technologies. The “unfit” alleles would eventually be lost from the population because genetic modification would prevent them from ever being passed on. Is it possible that one day in the near future clinicians and physicians will use CRISPR to decide which beneficial alleles get passed on? This would allow us to bypass natural selection in totality.
 |
| Image courtesy of Prezi |
Among similar lines, one study done at Yerkes in the Dias and Ressler lab opens yet another door to the realm of epigenetics and how ancestral sensitivity to certain stimuli can be transferred to subsequent generations. One notable example of this transgenerational information transfer includes the “Dutch Hunger Winter” in 1944, during which the F0 generation experienced the famine conditions and the F1 generation, in utero at that time, later developed astonishing rates of diabetes and obesity, as did their offspring (F2 generation). Similarly, another study found that a nutrition-linked mechanism through the male line seems to have influenced the risk for cardiovascular diseases and diabetes mellitus mortality. Taken further, maybe one day we can use CRISPR to edit our genes so that such unfavorable traits are not passed along in this way.
Might this be uniquely ethically complicated as we discuss editing genes related to brain function? One lab in China has genetically engineered more than 12 monkeys with a version of autism, in hopes of testing treatment for autism in humans. The reasoning for using monkeys in this study is that we share a more developed prefrontal cortex (a brain area where many psychiatric disorders seem to be impacted) and this should enable more generalizable results and translatable treatments.
Françoise Baylis, Canadian bioethicist and philosopher, posed the following questions. If you have a patient with Huntington’s, why treat just their symptoms, “allow them to reproduce and have children with Huntington’s, and we just have to do this over and over and over again? Why not fix it once and for all?!” This question of hers addresses the issues with the balance of “to do” or “not to do.”
Kevin Esvelt, an assistant professor at Harvard delves more into the delicate balance between the benefits and costs of using such technology and expands on the future directions for neurodiversity. He states, “if you give parents the option of zero autism risk in exchange for a certain loss of creativity or unusual talents, I expect almost all of them would take it — to the detriment of humanity.”
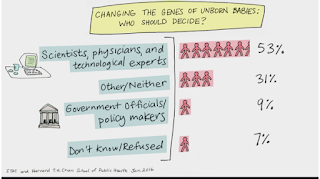
That brings me to my next question, that of responsibility. Whose decision is it? Who decides what a “beneficial gene” is and who all should receive such modifications? A recent poll conducted by Harvard T.H Chan School of Public Health, researchers asked about “changing the genes of unborn babies: who should decide?” The results indicated that 53% of responders said “scientists, physicians, and technological experts” while 9% said “government officials and policy makers” (31% answered neither, presumably the potential parents). Some argue that parents should have complete authority when it comes to gene editing, a process they likened to that of autonomy in choosing sperm donors. However, a contrasting view argues that “parental autonomy must be weighed against the interests of future generations who cannot consent to the genetic modifications their flesh will be heir to.”
Does this mean that the social injustice will deepen and the gap between the classes will widen or perhaps we’ll have an even more rampant view of “normalizing” and less appreciation of neurodiversity? According to one source, in Israel, their national health system provides its citizens with preimplantation genetic diagnoses, such that “any Israeli citizen can now fertilize multiple embryos with in vitro fertilization (IVF) and then choose to implant the one that seems to have the best genetic health.” But it’s unclear if everyone would agree what “best genetic health” would mean and how that would be interpreted by neurodiversity advocates.
Want to cite this post?
Sahu, S. (2017). A CRIPSR View of Life. The Neuroethics Blog. Retrieved on , from http://www.theneuroethicsblog.com/2017/01/a-crispr-view-of-life.html



Comments
Post a Comment